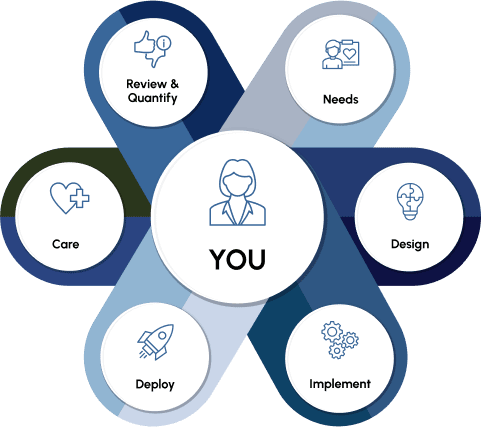
The New Pathway
Novir is establishing a new precare pathway focusing on shifting the traditional healthcare journey from symptoms to diagnosis to a preventive model that provides affordable and accessible screenings to anyone, anywhere. Our model focuses on detection, analysis, diagnosis, and therapy – stitching together the patient journey from end to end.